Atomic Structure
Matter is made up of tiny particles such as atoms, molecules, and ions.
Atoms
An atom is the smallest unit of an element that can participate in a chemical reaction.
Molecules
A molecule is the smallest part of a substance that can exist independently and still retain the chemical properties of that substance. Molecules are made up of atoms.
Atomicity
Atomicity refers to the number of atoms in a molecule of an element:
- Monatomic: 1 atom per molecule (e.g., noble gases)
- Diatomic: 2 atoms per molecule (e.g., O2, H2)
- Triatomic: 3 atoms per molecule (e.g., O3)
Ions
An ion is an atom or a group of atoms that carry an electric charge. Charged groups of atoms are called radicals.
Types of Radicals
Acid radicals are negatively charged groups of atoms that retain their identity. Examples include:
- SO42−
- NO3−
Cations and Anions
- Cations – positively charged ions (e.g., Ca2+, Na+, NH4+)
- Anions – negatively charged ions (e.g., CO32−, SO42−, Cl−, OH−)
Dalton’s Atomic Theory (1808)
John Dalton, a British scientist, proposed a theory about atoms which stated:
- All elements are made up of small, indivisible particles called atoms.
- Atoms cannot be created or destroyed in chemical reactions.
- Atoms of the same element are identical, but differ from those of other elements.
- Atoms of different elements combine in simple whole number ratios to form compounds.
- All chemical changes are due to the combination or separation of atoms.
Modifications to Dalton’s Theory
New discoveries in the 20th century led to updates to Dalton’s atomic theory:
- Not indivisible: Rutherford discovered that atoms are made of protons, neutrons, and electrons.
- Not always indestructible: In nuclear reactions, atoms can split, releasing a lot of energy (nuclear fission).
- Not always identical: Isotopes of the same element have the same proton number but different masses (e.g., chlorine isotopes).
- Not always simple combinations: Organic compounds like proteins and starch can have thousands of atoms per molecule, unlike simple inorganic compounds.
Thomson Model
Thomson proposed an atomic model that visualized the atom as a uniform sphere of positive charge with embedded negatively charged electrons. He also determined the charge-to-mass ratio (e/m) of electrons and found that this ratio remained constant for all cathode ray particles, regardless of the gas in the tube or the metal used.
Rutherford Model
Rutherford introduced a planetary atomic model, suggesting that the atom consists of a dense, positively charged core called the nucleus, where most of the atom's mass is concentrated. Surrounding this nucleus, negatively charged electrons orbit much like planets around the sun. To maintain electrical neutrality, the number of electrons must equal the number of protons in the nucleus.
Limitations of the Rutherford Model
- It predicted that atoms should emit light over a continuous range of frequencies, whereas experimental results showed discrete line spectra.
- It suggested that atoms should be unstable, with electrons spiraling into the nucleus. However, atoms are generally stable in reality.
- The model failed to explain experimental observations, leading to modifications introduced by Niels Bohr.
Bohr's Model
Bohr proposed a refined model of the hydrogen atom with the following key ideas:
Postulates of Bohr’s Model
- Electrons move around the nucleus in specific circular orbits called energy levels. The centrifugal force from this motion balances the electrostatic attraction between the electron and the nucleus. Electrons can move in these orbits without losing energy, and only certain discrete orbits are possible.
- The energy of an electron is quantized, meaning it can only take specific discrete values. Electrons do not lose energy continuously but make energy transitions in quantum jumps, emitting or absorbing energy in the form of photons.
- It explained why atoms emit line spectra and accurately predicted the wavelengths of hydrogen's spectral lines.
- It provided an explanation for absorption spectra, where photons of specific wavelengths excite electrons to higher energy levels.
- It explained atomic stability by stating that the ground state is the lowest energy state for an electron, preventing further energy loss.
- It correctly predicted the ionization energy of hydrogen as 13.6 eV.
- n = 1 for K shell
- n = 2 for L shell
- n = 3 for M shell
- n = 4 for N shell
- K shell (n=1): 2 × 1² = 2 electrons
- L shell (n=2): 2 × 2² = 8 electrons
- M shell (n=3): 2 × 3² = 18 electrons
- N shell (n=4): 2 × 4² = 32 electrons
- K shell (n=1) → 1 sublevel: 1s
- L shell (n=2) → 2 sublevels: 2s, 2p
- M shell (n=3) → 3 sublevels: 3s, 3p, 3d
- N shell (n=4) → 4 sublevels: 4s, 4p, 4d, 4f
- s-sublevel has 1 orbital (spherical in shape)
- p-sublevel has 3 orbitals (dumbbell-shaped)
- d-sublevel has 5 orbitals
- f-sublevel has 7 orbitals
- K shell: 1² = 1 orbital
- L shell: 2² = 4 orbitals
- M shell: 3² = 9 orbitals
- N shell: 4² = 16 orbitals
-
Principal Quantum Number (n):
Represents the main energy level or shell of an electron. It has whole number values such as 1, 2, 3, 4, and so on.
-
Subsidiary (Azimuthal) Quantum Number
(l):
Describes the shape of the orbital. It has integer values ranging from 0 to (n - 1).
-
Magnetic Quantum Number (m):
Indicates the orientation of an orbital in space. It can have integer values from –l to +l.
-
Spin Quantum Number (s):
Describes the direction of the electron's spin and can have values of –1/2 or +1/2.
Successes of Bohr’s Model
Structure of an Atom
An atom consists of three main particles: protons, neutrons, and electrons. In 1906, Rutherford discovered that the proton and neutron are located in the nucleus of the atom. Protons carry a positive charge, neutrons are neutral, and since the nucleus contains both, it is positively charged overall. Surrounding the nucleus are electrons, which are negatively charged and move in orbits. Atoms are electrically neutral because they have equal numbers of protons and electrons.
Properties of Fundamental Particles
| Particle | Mass | Charge |
|---|---|---|
| Proton | 1 unit | +ve |
| Neutron | 1 unit | Neutral |
| Electron | 1/1840 unit | -ve |
Distribution of Electrons in the Atom
Shells and Energy Levels
Electrons move continuously around the nucleus in specific paths called shells. These shells are labeled with capital letters: K, L, M, and N. Each shell corresponds to an energy level, represented by the principal quantum number (n), where:
The higher the value of n, the higher the energy level and the farther the shell is from the nucleus. The maximum number of electrons a shell can hold is given by the formula 2n²:
Sub-energy Levels and Atomic Orbitals
Each shell contains one or more sub-levels, labeled as s, p, d, and f. The number of sub-levels in a shell is equal to the principal quantum number (n):
Each sub-level contains orbitals, which are regions around the nucleus where there is a high probability of finding an electron:
The total number of orbitals in a shell is given by n²:
Each orbital can hold a maximum of two electrons.
Rules or Principles of Filling Electrons in Shells
1. Aufbau Principle
The Aufbau Principle states that electrons fill orbitals in order of increasing energy. Electrons will first occupy the orbital with the lowest energy before moving to higher energy orbitals. "Aufbau" is a German word meaning "building up." The diagram (not shown here) illustrates the increasing order of orbital energy levels.
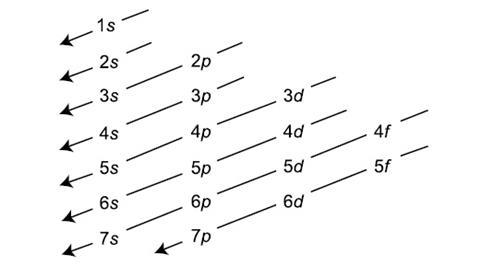 Credit:
https://edu.rsc.org
Credit:
https://edu.rsc.org
2. Pauli's Exclusion Principle
According to Pauli's Exclusion Principle, no more than two electrons can occupy the same orbital. When two electrons share an orbital, they must have opposite spins.
3. Hund's Rule of Maximum Multiplicity
Hund's Rule states that electrons will occupy degenerate orbitals (orbitals of the same energy) singly before any pairing occurs. This means electrons prefer to have parallel spins in different orbitals before pairing up. An example of degenerate orbitals is the three orbitals in the 2p sublevel, which all have the same energy.
Note: When electrons fill energy sublevels according to the Aufbau Principle, the arrangement is called the ground state electronic configuration. This is the most stable form of an atom.
Quantum Numbers
Research shows that the energy and behavior of an electron can be described using four quantum numbers: